A longer version of this story appears at Marijuana Business Daily International.
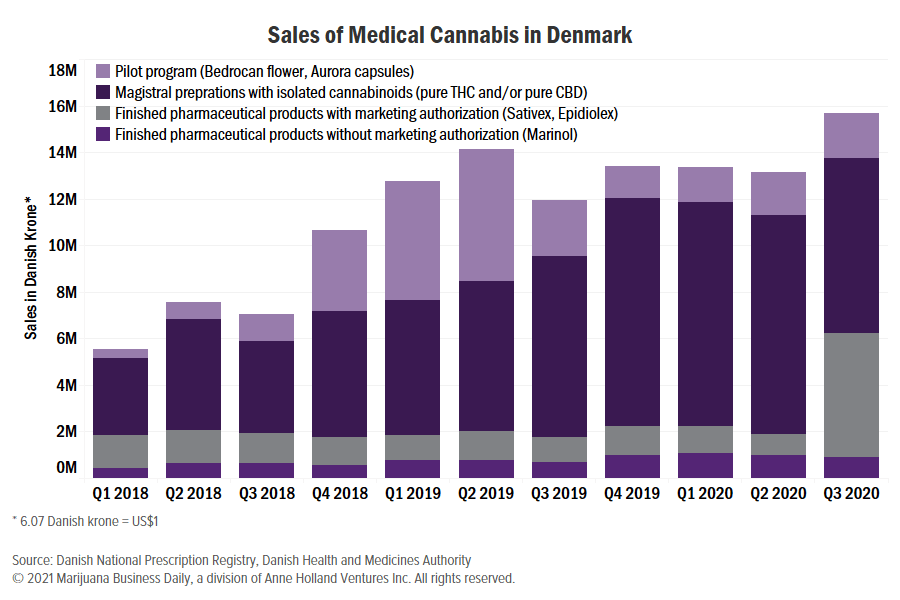
Sales of medical marijuana in Denmark posted healthy gains in the third quarter of 2020, coinciding with the launch of GW Pharmaceuticals’ CBD drug Epidiolex.
Overall sales of all medial cannabis products grew from about 13 million Danish Krones ($2 million) in the second quarter to almost 16 million Danish Krones in the third quarter, representing a gain of more than 20%.
But revenue within the country’s pilot program, which allows sales of flower and oil without marketing authorization, remains small and far from the peak seen one year ago.
In the third quarter of 2020, only 12.5% of total revenue generated by medical cannabis sales came from products sold as part of the pilot program, official data shows.
That is down from early 2019, when transactions in the pilot program represented 40% of the total medical cannabis sales.
The biggest change from the previous quarter was a meaningful increase of sales in the category of finished pharmaceutical products with marketing authorization, explained by the start of GW Pharmaceuticals’ Epidiolex sales.
The category of magistral preparations – medicines prepared in a pharmacy from isolated THC and/or CBD – decreased coinciding with the start of Epidiolex sales, a sign that some CBD prescriptions switched from magistral preparations to Epidiolex, a finished pharmaceutical product.
Accounting for all medical cannabis products, total sales during the third quarter were about 15.7 million Danish krone, of which:
- The pilot program represented only 12.5% of the total.
- Magistral preparations with pure THC or pure CBD – no full-spectrum products – accounted for almost half of all sales.
- A third of the sales corresponded to finished pharmaceutical products with marketing authorization. Almost all the revenue in this category was generated by Epidiolex sales, which started in mid-2020; the rest are attributable to GW Pharmaceuticals’ other registered medicine, Sativex.
- The remaining 6% corresponded to other finished pharmaceutical products that do not have marketing authorization in Denmark, mainly U.S. Food and Drug Administration-approved Marinol.
Considering all types of products, 6,130 patients received a prescription for medical cannabis at least once from January 2018 until September 2020 – most for isolated cannabinoids magistral preparations.
Alfredo Pascual can be reached at [email protected]